Azobenzene Polymers for Photonic Applications
Citation
Yager, K.G.; Barrett, C.J. "Azobenzene Polymers for Photonic Applications"
Smart Light-Responsive Materials: Azobenzene-Containing Polymers and Liquid Crystals, Zhao, Y. and Ikeda, T., eds. John Wiley and Sons 2008,
Chapter 1 ISBN 978–0–470–17578–1.
doi: 10.1002/9780470439098.ch1Summary
We provide a general introduction to the broad class of materials based on the 'azobenzene' chemical motif.
Abstract
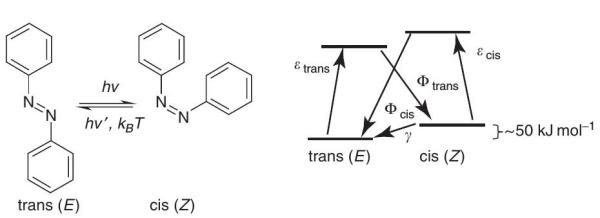
Azobenzene, with two phenyl rings separated by an azo (–N=N–) bond, serves as the parent molecule for a broad class of aromatic azo compounds. These chromophores are versatile molecules, and have received much attention in research areas both fundamental and applied. The strong electronic absorption maximum can be tailored by ring substitution to fall anywhere from the ultraviolet (UV) with the to visible fact that red these regions, azo G allowing groups are chemical relatively fine-tuning robust and of color. chemically This,stable, combined has prompted extensive study of azobenzene-based structures as dyes and colorants. The rigid mesogenic R shape of the molecule is well suited to spontaneous organization into liquid crystalline (LC) phases, and hence polymers doped or functionalized with azobenzene-based chromophores (azo polymers) are common as LC media. P With appropriate electron-donor–acceptor ring substitution, the p electron delocalization of the extended aromatic structure can yield high optical nonlinearity, and zo chromophores have seen extensive study for nonlinear optical applications C as well. One of the most interesting properties of these chromophores however, and the main subject of this review, is the readily induced and reversible isomerization about the azo bond between the trans and cis geometric isomers and the geometric changes that result when azo chromophores are incorporated into polymers and other materials. This light-induced interconversion allows systems incorporating azobenzenes to be used as photoswitches, effecting rapid and reversible control over a variety of chemical, mechanical, electronic, and optical properties.
Perhaps of a range as wide as the interesting phenomena displayed by azo aromatic compounds is the variety of molecular systems into which these chromophores can be incorporated. In addition to LC media and amorphous glasses, azobenzenes can be incorporated into self-assembled monolayers and superlattices, sol–gel silica glasses, and various biomaterials. The photochromic or photoswitchable nature of azobenzenes can also be used to control the properties of novel small molecules, using an attached aromatic azo group. A review will be presented here of the photochemical and photophysical nature of chromophores in host polymers, the geometric and orientational consequences of this isomerization, and some of the interesting ways in which these phenomena have been expolited recently to exert control over solution and biochemical properties using light. This photoisomerization can be exploited as a photoswitch to orient the chromophore (which induces birefringence), or even to perform all-optical surface topography patterning. These photomotions enable many interesting applications, ranging from optical components and lithography to sensors and smart materials.