Nanoconfinement and Salt Synergistically Suppress Crystallization in Polyethylene Oxide
Citation
Zhang, Z.; Ding, J.; Ocko, B.M.; Lhermitte, J.; Strzalka, J.; Choi, C.-H.; Fisher, F.T.; Yager, K.G.; Black, C.T. "Nanoconfinement and Salt Synergistically Suppress Crystallization in Polyethylene Oxide"
Macromolecules 2020,
53 1494–1501.
doi: 10.1021/acs.macromol.9b01725Summary
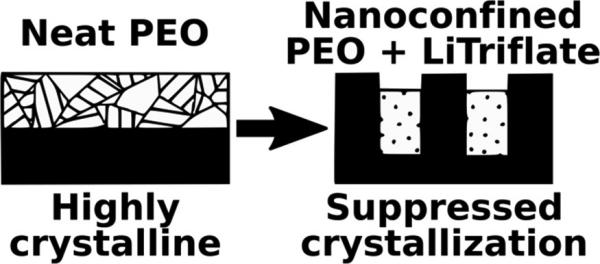
We show how combining salt addition and nano-confinement can be used to suppress the crytallization of PEO.
Abstract
Suppressing the crystallization of polyether-based solid electrolytes is a widely sought-after strategy to improve ionic conductivity. We report the effects of nanoconfinement on polyethylene oxide electrolytes. We find that neat polyethylene oxide responds to nanoconfinement by adopting a preferred orientation yet is able to crystallize even in nanoconfinement volumes with widths as small as 8 nm. However, the combination of nanoconfinement and salt addition does suppress polymer crystallization at room temperature even though either factor alone cannot. Such synergistic suppression of crystallization has implications for polymer electrolytes since amorphous rather than crystalline domains predominantly contribute to ionic conduction. Our results suggest that salts previously discounted due to their inability to suppress crystallinity in bulk materials could be made viable when combined with nanoconfinement, thereby opening new possibilities for high-performance solid polymer electrolytes.

