Excited-State Processes in Slow Motion: An Experiment in the Undergraduate Laboratory
Citation
Galley, W.C.; Tanchak, O.M.; Yager, K.G.; Wilczek-Vera, G. "Excited-State Processes in Slow Motion: An Experiment in the Undergraduate Laboratory"
Journal of Chemical Education 2010,
87 1252–1256.
doi: 10.1021/ed100285wSummary
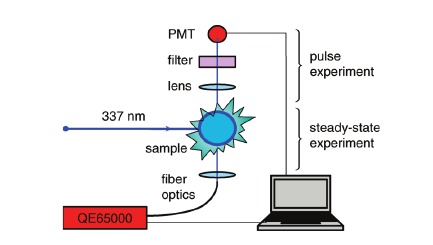
The details for an experiment in the undergraduate physical chemistry laboratory are presented. Excited-state processes can be studied in detail using a laser pump/probe setup.
Abstract
Lasers have transformed chemistry and the everyday world. Therefore, it is not surprising that undergraduate chemistry students are frequently exposed to fairly advanced laser techniques. The usual topics studied with lasers are molecular spectroscopy and chemical kinetics. Static and dynamic fluorescence experiments seem to be particularly popular. The phenomenon of phosphorescence, on the other had, has received much less attention in the undergraduate physical chemistry laboratory. A few years ago, we developed a laser experiment that introduced students to the phosphorescence quenching of the carbazole?naphthalene system under steady-state and pulse-excitation conditions. The purpose of that experiment was to demonstrate the experimental and theoretical aspects of triplet?triplet nonradiative energy transfer between two aromatic molecules. Subsequently, we modernized the experiment by introducing an inexpensive and easy-to-use Ocean Optics data acquisition system. This not only greatly simplified the experiment, but also made it more accessible to students. This article presents the upgraded version of the experiment together with suggestions for practical implementation.

